- Seebeck | Peltier | Thomson | Joule effects
- Generate electricity thanks to chemistry
- Alternative solution: solar and photovoltaic panels
- Quiz
Generate electricity thanks to Seebeck | Peltier | Thomson | Joule effects
Seebeck effect
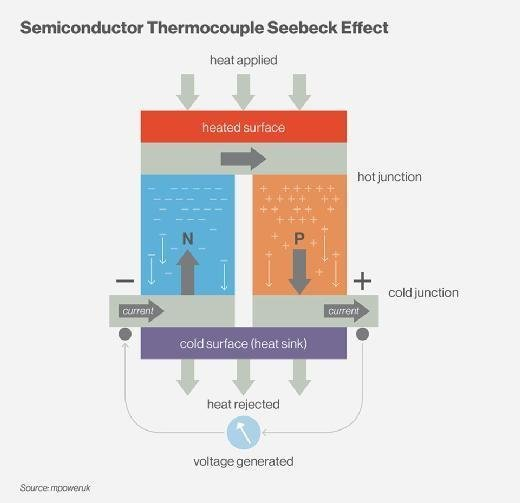
Thomas Johann Seebeck discovered in 1821 that a circuit consisting of two dissimilar metals with connections at different temperatures could deflect a compass magnet. Seebeck assumed it was due to magnetism caused by the temperature differential at first. However, it was rapidly understood that the induced current is an electrical current, which deflects the magnet according to Ampere’s law. In a closed circuit, the temperature differential creates an electric potential (voltage) that can generate an electric current. This is known as the Seebeck effect today. The difference in temperature between the two junctions determines the voltage generated. The Seebeck coefficient, often known as the thermoelectric power or thermopower, is the proportionality constant (a) and can be expressed in V.K-1, and characterizes the voltage resulting from a deviation of 1 K.
Metals generally have Seebeck coefficients of only a few μV.K-1. Semiconductors, on the other hand, have slightly higher Seebeck coefficients due to their low thermal conductivity.
The Seebeck effect is the opposite of the Peltier effect.
Peltier Effect
Jean Charles Athanase Peltier, a French watchmaker and part-time scientist, discovered in 1834 that an electrical current may cause heating or cooling at the junction of two different metals. In 1838, Lenz demonstrated that heat could be withdrawn from a junction to freeze water into ice, or heat could be created to melt ice, depending on the direction of current flow. The electrical current determines how much heat is absorbed or generated at the junction. The Peltier coefficient is the proportionality constant.
Thomson effect
The effect is named after James Prescott Joule and William Thomson, 1st Baron Kelvin, who discovered it in 1852. It followed upon earlier work by Joule on Joule expansion, in which a gas undergoes free expansion in a vacuum and the temperature is unchanged, if the gas is ideal.
The Thomson effect depends both on the temperature gradient and charge current across the material. Unlike the Peltier and Seebeck effects, the Thomson effect does not require the presence of two materials—it can also occur in a homogenous slab of one substance. Originally proposed by William Thomson (also known as Lord Kelvin), the Thomson effect links together the Peltier coefficient Π (the heat absorbed/evolved per unit charge) and the Seebeck coefficient S (the voltage generated per unit temperature difference) at any temperature T0, using Π=ST0Π=ST0 and the Thomson coefficient τ=S⋅dS∕dT
Joule effect
Thomson Joule is one of the most important founders of thermodynamics. In 1843, he succeeded in giving a strict formulation of the principle of the conservation of energy, and he undertook to quantify the relations between heat and work by means of very diverse experiments of extraordinary precision. In 1852, Joule William Thomson discovered that when a gas expands adiabatically, the temperature of the gas drops. This phenomenon was called the “Joule-Thomson effect” and was used to build a large cooling industry in the 19th century.
His study of the nature of heat and his discovery of the relationship with mechanical work led him to the theory of conservation of energy (the first law of thermodynamics). He also formulated a relationship between the electric current flowing through a resistor and the heat dissipated by it, known since the 20th century as Joule’s law.
How does it works?
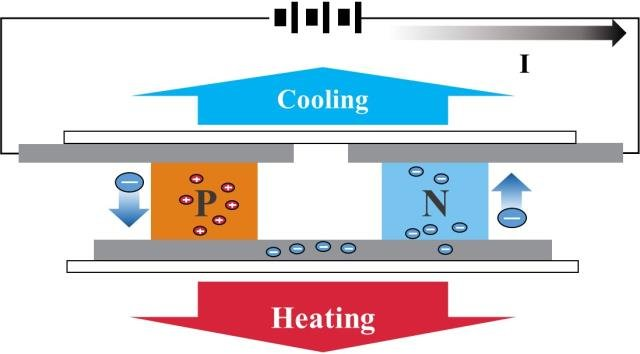
A continuous current must flow through a succession of semiconductors which have the property of being either good or bad conductors connected by copper connections, the whole being caught between two heat-conducting plates.
It will be observed that one of the two positively charged electron plates (less electrons) will become hot and the second negatively charged electron plate (more electrons) will become cold.
These semiconductors acting as thermocouples allow the displacement of this energy in the form of heat, they are generally made of bismuth tellurium, elements best suited for operation at room temperature.
The main difficulty of the Peltier effect is to be able to evacuate the heat emitted by the Joule effect without disturbing the production of cold, the more we will not have the capacity to evacuate the heat the more the refrigeration production will be important.
The Peltier effect is related to entropy transport by the charge carriers (electrons or holes) inside the material. Thus, when there is a heat absorption in a given place and a release in another place, this is due to the fact that the electrons or holes lose their entropy by passing from a material to a material b (so there is a release of heat), whereas conversely, the Seebeck effect, they find entropy by passing from matter b to matter a (so there is heat absorption), because there is conservation of energy, it is the first principle of thermodynamics.