You don’t know if it’s possible to generate electricity to power a small electrical device close to a heater?
Our MOOC is made for you !
Table of contents :
- Generate electricity thanks to Seebeck/Peltier/Thomson effects
- Generate electricity thanks to chemistry
- Alternate solution : solar panel
- Conclusion
- Quiz
Generate electricity thanks to Seebeck/Peltier/Thomson effect
Peltier Effect
Jean Charles Athanase Peltier, a French watchmaker and part-time scientist, discovered in 1834 that an electrical current may cause heating or cooling at the junction of two different metals. In 1838, Lenz demonstrated that heat could be withdrawn from a junction to freeze water into ice, or heat could be created to melt ice, depending on the direction of current flow. The electrical current determines how much heat is absorbed or generated at the junction. The Peltier coefficient is the proportionality constant.
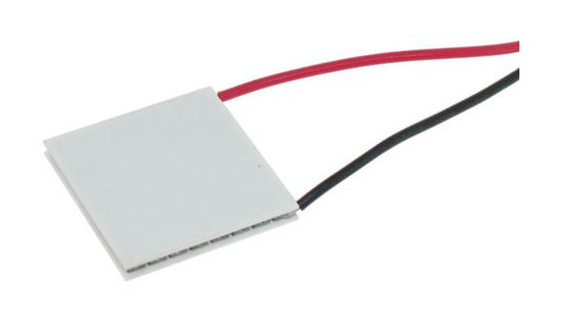
Seebeck effect
Thomas Johann Seebeck discovered in 1821 that a circuit consisting of two dissimilar metals with connections at different temperatures could deflect a compass magnet. Seebeck assumed it was due to magnetism caused by the temperature differential at first. However, it was rapidly understood that the induced current is an electrical current, which deflects the magnet according to Ampere’s law. In a closed circuit, the temperature differential creates an electric potential (voltage) that can generate an electric current. This is known as the Seebeck effect today. The difference in temperature between the two junctions determines the voltage generated. The Seebeck coefficient, often known as the thermoelectric power or thermopower, is the proportionality constant (a) and can be expressed in V.K-1, and characterizes the voltage resulting from a deviation of 1 K.
Metals generally have Seebeck coefficients of only a few μV.K-1. Semiconductors, on the other hand, have slightly higher Seebeck coefficients due to their low thermal conductivity.
The Seebeck effect is the opposite of the Peltier effect.
Thomson effect
The effect is named after James Prescott Joule and William Thomson, 1st Baron Kelvin, who discovered it in 1852. It followed upon earlier work by Joule on Joule expansion, in which a gas undergoes free expansion in a vacuum and the temperature is unchanged, if the gas is ideal.
The Thomson effect depends both on the temperature gradient and charge current across the material [4]. Unlike the Peltier and Seebeck effects, the Thomson effect does not require the presence of two materials—it can also occur in a homogenous slab of one substance. Originally proposed by William Thomson (also known as Lord Kelvin), the Thomson effect links together the Peltier coefficient ΠΠ (the heat absorbed/evolved per unit charge) and the Seebeck coefficient SS (the voltage generated per unit temperature difference) at any temperature T0T0, using Π=ST0Π=ST0 and the Thomson coefficient τ=S⋅dS∕dT
Joule effect
Thomson Joule is one of the most important founders of thermodynamics. In 1843, he succeeded in giving a strict formulation of the principle of the conservation of energy, and he undertook to quantify the relations between heat and work by means of very diverse experiments of extraordinary precision. In 1852, Joule William Thomson discovered that when a gas expands adiabatically, the temperature of the gas drops. This phenomenon was called the “Joule-Thomson effect” and was used to build a large cooling industry in the 19th century.
His study of the nature of heat and his discovery of the relationship with mechanical work led him to the theory of conservation of energy (the first law of thermodynamics). He also formulated a relationship between the electric current flowing through a resistor and the heat dissipated by it, known since the 20th century as Joule’s law.
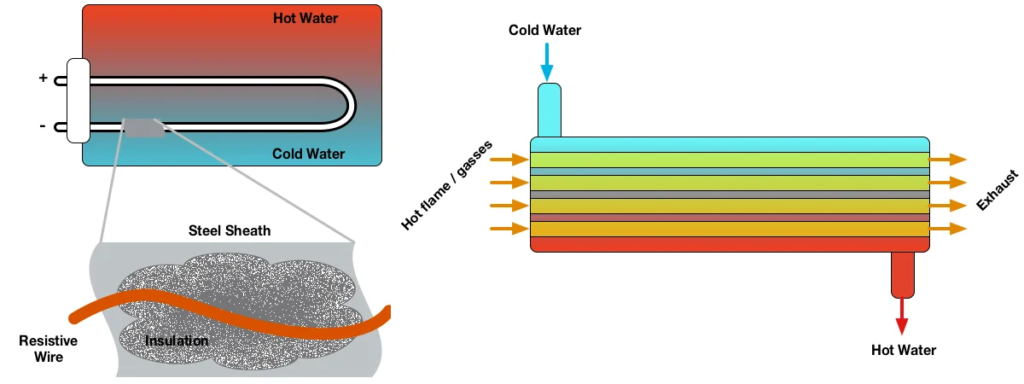
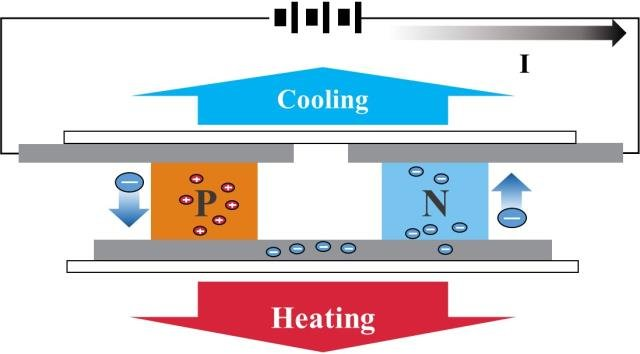
How does it works?
A continuous current must flow through a succession of semiconductors which have the property of being either good or bad conductors connected by copper connections, the whole being caught between two heat-conducting plates.
It will be observed that one of the two positively charged electron plates (less electrons) will become hot and the second negatively charged electron plate (more electrons) will become cold.
These semiconductors acting as thermocouples allow the displacement of this energy in the form of heat, they are generally made of bismuth tellurium, elements best suited for operation at room temperature.
The main difficulty of the Peltier effect is to be able to evacuate the heat emitted by the Joule effect without disturbing the production of cold, the more we will not have the capacity to evacuate the heat the more the refrigeration production will be important.
The Peltier effect is related to entropy transport by the charge carriers (electrons or holes) inside the material. Thus, when there is a heat absorption in a given place and a release in another place, this is due to the fact that the electrons or holes lose their entropy by passing from a material to a material b (so there is a release of heat), whereas conversely, the Seebeck effect, they find entropy by passing from matter b to matter a (so there is heat absorption), because there is conservation of energy, it is the first principle of thermodynamics.
Is it doable for a radiator
The initial objective of this MOOC was to educated students on how to electrically aliment a radiator isolated from the power grid. So, it’s important to check if this solution could work for a radiator.
From our analysis of the subject, it seems impossible to do so, indeed the different effects mentioned hereinbefore are used to cool system where heat is a waste, this is a way in those case to revalorize the energy that would otherwise be dissipated in the air. One of such systems where the effects mentioned above could be use is using exhaust gazes to generate electricity.
So, in other words, you could think of those effects as similar to regenerative braking for electric motors, you will generate way less energy than what you needed to increase the speed of your motor, but you would at least recharge a bit.
There is multiple issue that make it quite hard to work for a radiator:
- Heat is not a waste (so you don’t want to capture it)
- You need a temperature difference in order to generate electricity
- The heat of the radiator is just too low to generate any usable electricity
To conclude about it, it’s feasible for cooling radiators but not for heating radiators which are the one that we would like it to work.
Generate electricity thanks to chemistry
Ultra-confined liquids or Solid thermoelectric
Solid thermoelectric materials convert a temperature difference into electrical energy. They are thus an important energy resource for years to come. However, the most efficient materials are rare, expensive and often toxic.
However, an alternative possibility exists and consists in the use of nanofluidic channels confining salt water. Such systems have attracted much attention recently, as they are capable of producing electricity from the osmotic energy of seawater. This «blue energy» comes from the phenomenon of osmosis, that is, the spontaneous flow of the liquid from the most concentrated medium to the least concentrated. But the application of these devices to the electricity recycling of waste heat from many industrial processes in electricity is only now beginning to be studied. This lower interest can be explained by the standard image of the thermoelectricity of charged liquids, developed in the 1980s, which predicted performances far below those of thermoelectric materials.
This original approach, transposable to materials of very varied shapes and natures, provides a theoretical framework to predict the unusual behavior of charged liquids, especially in contact with nanoporous metal structures, with direct applications in the field of energy, environment or chemistry.
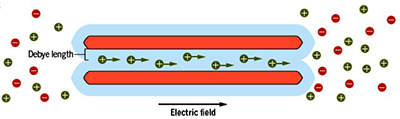
How does it works?
By circulating liquids in charged nanoscale channels, it is possible to convert heat into electricity as efficiently as the best thermoelectric materials.
The scientists tested these models by simulating the behaviour of the material at the atomic level. In this type of simulation, the motion of each atom is described explicitly, which makes it possible to measure independently the influence of the various parameters (interactions with the walls, electrostatic contribution) on the movement of atoms and therefore of the electric current. Against all odds, they showed that the performances of nanofluidic systems were a hundred times higher than the predictions of standard models, and could be comparable to those of the best solid thermoelectric materials. This work demonstrates the potential of nanofluidic systems and, by helping to understand their mechanisms, can serve as a guide for the development of high-performance devices, an economical and non-toxic alternative to thermoelectric materials.
When subjected to a temperature difference, a nanofluidic channel can generate electricity, with a yield comparable to that of the best thermoelectric solids.
Is it doable for a radiator?
Chemical energy. On a small scale, energy storage for electrical use consists mainly of electrochemical storage (batteries and batteries) and electrical storage (capacitors and supercapacitors)
At present, chemistry does not allow us to create and store energy in sufficient quantities and in a continuous way without a source of raw material or an external source of energy at a low economic cost. The problem is that chemical chain reactions must be carried out, which implies the use of specialized equipment and the attention of qualified personnel.
About the futur
Scientists have introduced a new atomic-scale simulation method that describes the containment or adsorption of a charged fluid near a surface, taking into account the effects of electronic relaxation, in other words, the movement of electrons towards electric charges approaching the material. The approach consists in mimicking the effects of electron clustering against the charges of the liquid – the electrostatic screen – for any situation between an insulating material and a perfect metal. For this, Coulomb interactions are described via a “virtual” Thomas–Fermi fluid made of light and fast charged particles that imposes an electrostatic screen by reorganizing in the presence of the confined liquid. Using this strategy on a nano-confined ionic liquid, there is a wetting transition depending on whether the confining material is insulating or metallic.
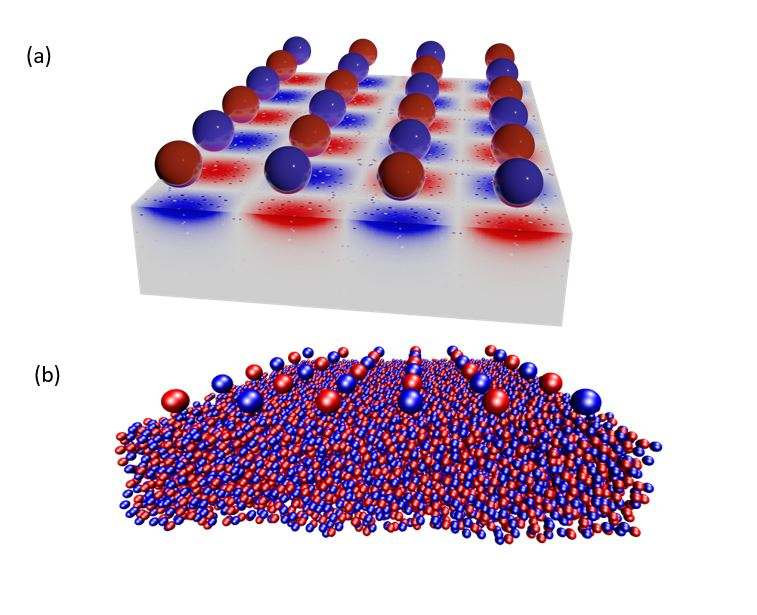
Used in energy storage, electrochemistry and catalysis, liquids with polar ions or molecules exhibit unexpected behaviors in the vicinity of a solid surface, especially when confined. For example, there is an unexplained link between the solidification temperature of the charged liquid and the metallic or insulating nature of the surface. The complexity of electrostatic interactions in confined geometry is a more active research topic than ever, but despite this, the modelling of ionic fluids at the interfaces remains partial and some of the observed behaviors still escape theoretical understanding. While most approaches assume perfectly insulated or metallic surfaces, the difficulty is to simulate the reaction of a surface in the case of real materials, whose electrostatic response is mostly intermediate between insulation and metal.
Alternate solution : solar panel
History
During the 19th century, the possibility of using the energy of the sun to meet the energy needs of human society took place in the literature of Emile Zola.
Towards the end of the book, the narrator notes that the depletion of coal mines is an important problem in view of the ever greater need to produce electricity. This is how he envisions the possibility of capturing solar energy in order to make it a “universal engine”.

How does it works?
Solar energy is captured through modules (large rectangles) covered with silicon cells, a semiconductor material. The capture and transformation of solar energy takes place as follows:
Photons (sunlight) strike the cells.
They transmit their energy to the electrons contained in the panels.
The electrons move and produce a direct electric current.
The inverter then converts the direct current into alternating current and makes it usable on the electrical grid or in a building.
Electricity can be used to power the household’s electrical appliances, sold to an electricity distributor or stored in a battery for later use in a building (for example, in winter, when there is no more sun).
Photovoltaic energy
The photovoltaic panel is composed of several photovoltaic cells. These are essentially silicon, which is the most abundant mineral chemical after oxygen in the Earth’s crust.
Photovoltaic cells thus capture solar rays and transform it into direct current. Thanks to a photovoltaic inverter, this direct current is then converted into alternating current.

Where can we install them?
Solar panels are traditionally installed on the roofs of houses or buildings. The efficiency of solar panels is optimized when the year-round sunshine is high and the roof:
- is facing due south;
- offers a tilt between 30 and 35 degrees;
- has no grey area.
- windows adjacent to the radiators
Depending on the size of the land and the energy requirements of the site, they can also be installed on ground supports. For example, it is possible to attach solar panels to a garden shed or to invest in a solar shade that can be used to protect a vehicle from the elements.
Further information on solar energy:
Solar energy is clean and renewable. The Earth receives more than 10,000 times as much sunlight as humanity consumes. Indeed, the surface of the globe receives annual solar energy with a power ranging from 85 to 290 W/m21. And this energy is inexhaustible because it is sustained by the nuclear reactions that take place in the sun. Although the resource has always existed, its use is fairly recent in the history of humanity.
In fact, there are two types of panels:
Solar panel converts solar radiation into heat
A solar panel is installed when you want to enjoy hot water in your home. Sometimes it is also used for heating. In its mode of operation, this model uses solar radiation to convert it into heat. The panel captures heat from the sun and transmits it into the energy transporter (heat transfer fluid) which passes through tubes. The heat then goes into a heat exchanger. The heat exchanger heats the water and stores it in a tank.
A photovoltaic panel has solar-ray sensor cells called “photovoltaic”. The energy drawn from the rays will be converted into electrical current. The electricity produced is ready to use, but can also be stored in batteries. Photovoltaic solar panels are a true ecological solution for the production of electricity. They do not emit greenhouse gases and the materials used are recyclable.
Is it doable for a radiator?
Will look horrible and is not efficient to have 1 panel per radiator, you would have a better time linking every radiator to the power grid (would look better and would be cheaper and you would be able to have more efficient solar panels being mounted on the roofs or whatever is the most suited instead of on every window close to a radiator….). But of course it fully depends on the total number of radiator you want to get electricity to. Having one radiator power by a single mini-solar panel is not a bad idea but having 200 powered by 200 different solar panels is a bad idea.
Conclusion
To conclude this MOOC on how to power isolated radiators with electicity, we wanted to adress what is for us the best choice out of the solution proposed:
The thermocouples based on Seebeck Effect/Thomson Effect are not efficient and will absorb the heat of the radiators, the chemical solution is unusable without regular and costly maintenance and the solar panels solution will require to link every radiators to the power grid…
So, if you want to power hot water/steam radiator isolated from the power grid, the best solutions, in our mind is to simply link them to the power grid…
This opinion is of course subject to critics and depends fully on the number of radiators you want to power. Indeed if you only have a few, having a mini solar panel can be viable but that wouldn’t be the case when you have hundreds of them to power.
